The atomic number is 21 which means that scandium has 21 protons. How many neutrons are in an ion.

How To Find The Number Of Neutrons In An Atom 11 Steps
The easiest way to find the number of protons neutrons and electrons for an element is to look at the elements atomic number on the periodic table.
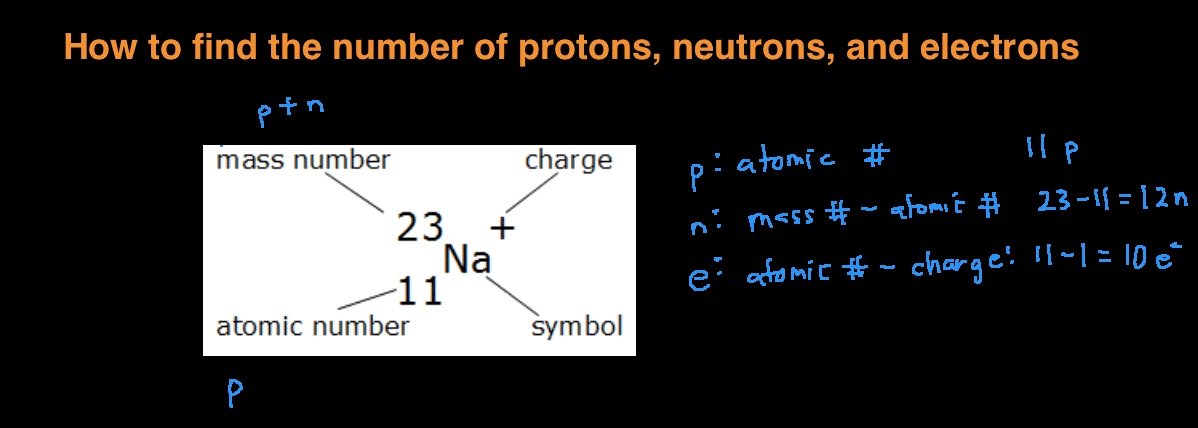
How do i find the number of protons. You can simply subtract the atomic number from the mass number in order to find the number of neutrons. Mass Number is the number of protons and neutrons in an isotope. Your mass number is the total number of neutrons and protons within the atom.
Protons and Electrons Problem. To find the number of neutrons you will need to subtract the atomic number from the atomic mass. Atomic number the number of a chemical element in the periodic system whereby the elements are.
Identify the number of protons and electrons in the Sc 3 ion. Not only does it show where it would be located on the periodic table it tells us how many protons are in t. Your atomic number is the amount of protons within the atom.
You must know chemical formula of compound so you can determine the number of atoms and type of atoms in terms of which molecular formula is written. Atomic Mass is the mass of the entire atom of an isotope. Use the Periodic Table to find the atomic number of Sc.
The number of protons determines an elements atomic number and is used to distinguish one element from another. The number will tell you how many protons make up a single atom of an element. The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element.
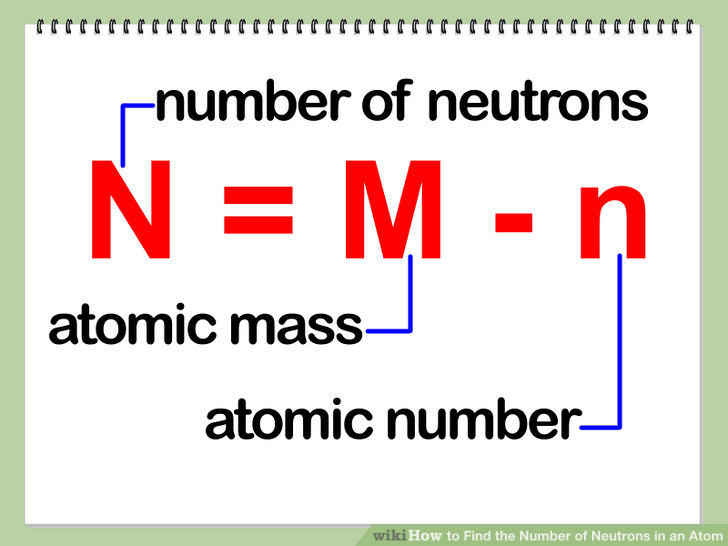
To learn how atoms form ions click here. This chemistry video tutorial explains how to calculate the number of protons neutrons and electrons in an atom or in an ion. Because neutrons and protons both have a mass of about 1 amu the difference between the mass number 210 and the atomic number 82 is equal to the number of neutrons in the nucleus of the atom.
A negatively-charged ion or anion has more electrons than protons. So to get the number of electrons you must add the size of charge to the atomic or proton number. It also explains the differe.
The number of electrons in a neutral atom is equal to the number of protons. We use the mass number in naming isotopes like Carbon-12 or Oxygen-17. That number is equal to the number of protons.
2122 A modern perspective has a proton composed of the valence quarks up up down the gluons and transitory pairs of sea quarks. Lastly the charge is on the upper right corner. How do you find the number of protons and electrons in an ion.
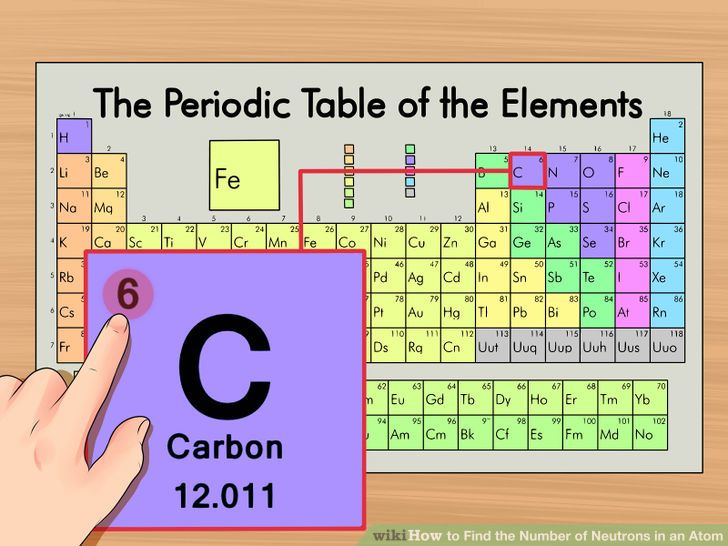
If there isnt any number or signs then it means that atom has no charge and is. The first step of how to find the number of neutrons in an isotope is to discover the element on the periodic table. Find the element on the periodic table.
Remember that the atomic number is the same as the number of protons. If the charge is positive there are more protons than electrons. The proton number is the atomic number of the element while the electron number is the atomic number minus the charge.
Locate the elements atomic number. The ion therefore contains 128 neutrons. The number in the top left is called the atomic number.
Together the number of protons and the number of neutrons determine an elements mass number. This is a whole number. Periodic Table Basics Learn how to use information from the periodic table to find the number of protons neutrons and electrons of an element.
Determine the number of electrons. I am showing how to find proton neutron and electron numbers of atom and ions in this video. You can find the number of neutrons if you know the isotope of the atom.
If the atom is neutral the number of electrons will be equal to the number of protons. Lets take an example of carbon dioxide. Protons are spin-1 2 fermions and are composed of three valence quarks making them baryons a sub-type of hadronsThe two up quarks and one down quark of a proton are held together by the strong force mediated by gluons.
What is atomic number short answer. Lets first talk about the numbers associated with the element and what they mean. An ion has an unequal number of protons and electrons.
The number on the upper left corner is the mass number which is equal to the neutrons and protons added together. Because the non-isotopic form of carbon-14 is only carbon C you need to locate the carbon on the periodic table. So from figure 3 the number of electrons for chloride ion is 17 1 18 electrons.
Number of protons is the atomic number. The number of electrons is the atomic number added to the charge. For example we will look at the carbon-14 isotope.
A positively-charged ion or cation has more protons than electrons. A neutral atom has the same number of protons and electrons charges cancel each other out. If the charge is negative electrons are in excess.
The number on the bottom left corner is the atomic number which tells you the number of protons. Again the number of protons is the atomic number. Find your element on the periodic table.
Protons are particles in the nucleus of an atom that has a positive charge equal to 1. How to calculate the number of electrons in an ion. Answer 1 of 4.

How To Find The Number Of Protons Neutrons And Electrons
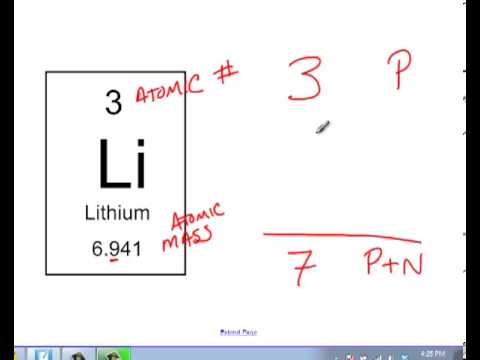
Calculating Number Of Neutrons Youtube
The Mass Number Of A Chromium Atom Is 52 And It Has 24 Protons How Many Neutrons Does This Atom Have Socratic
How Can We Find The Number Of Protons And Electrons Present In A Neutral Atom Socratic

How To Calculate The Number Of Protons Neutrons And Electrons Chemistry Youtube
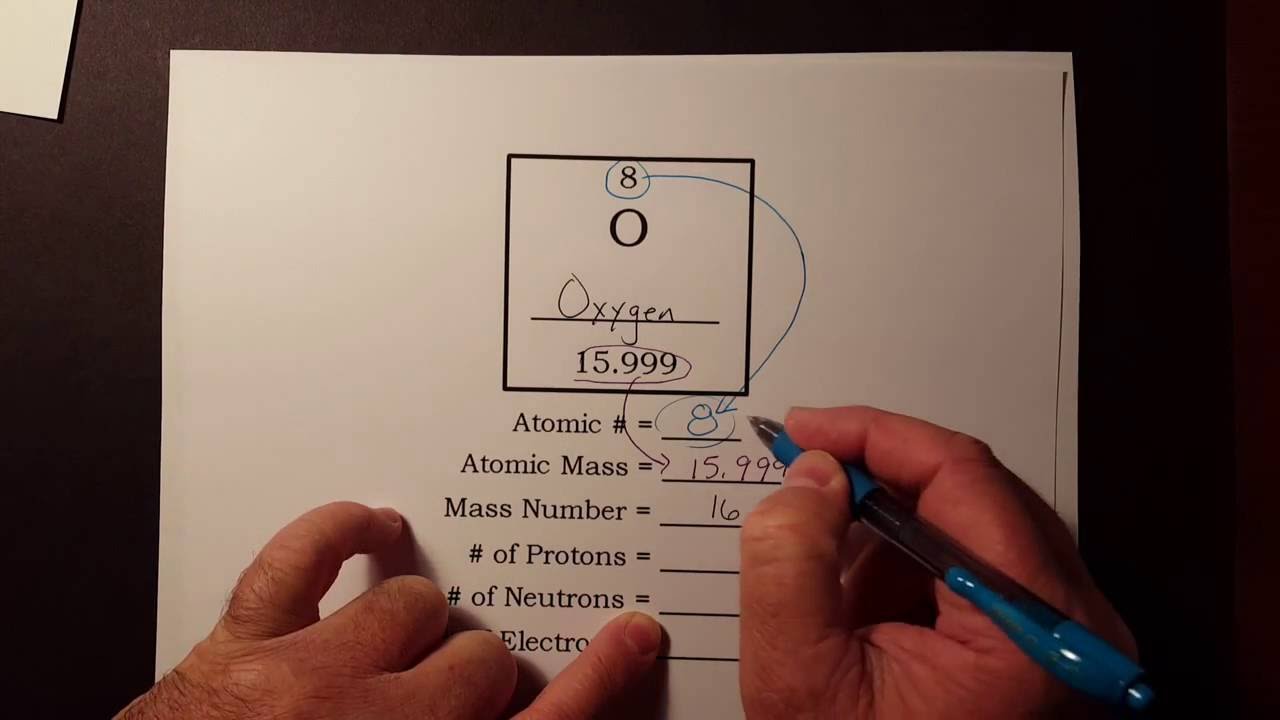
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube

How To Find The Number Of Neutrons In An Atom 11 Steps
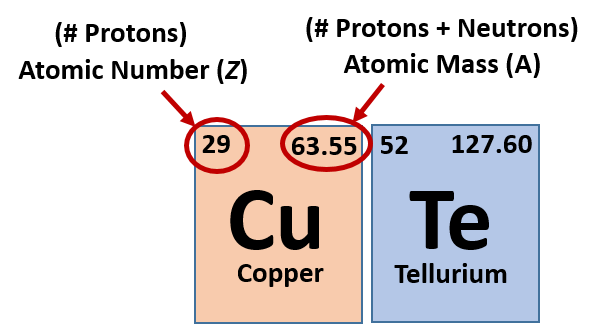
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
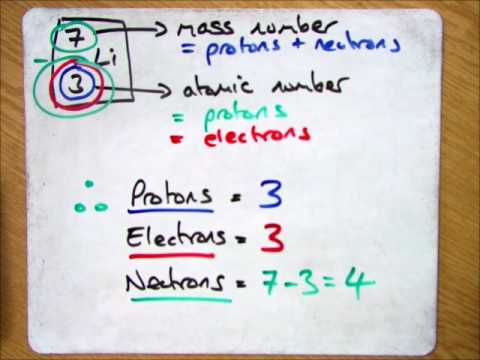
Calculating The Protons Neutrons And Electrons For An Atom Youtube
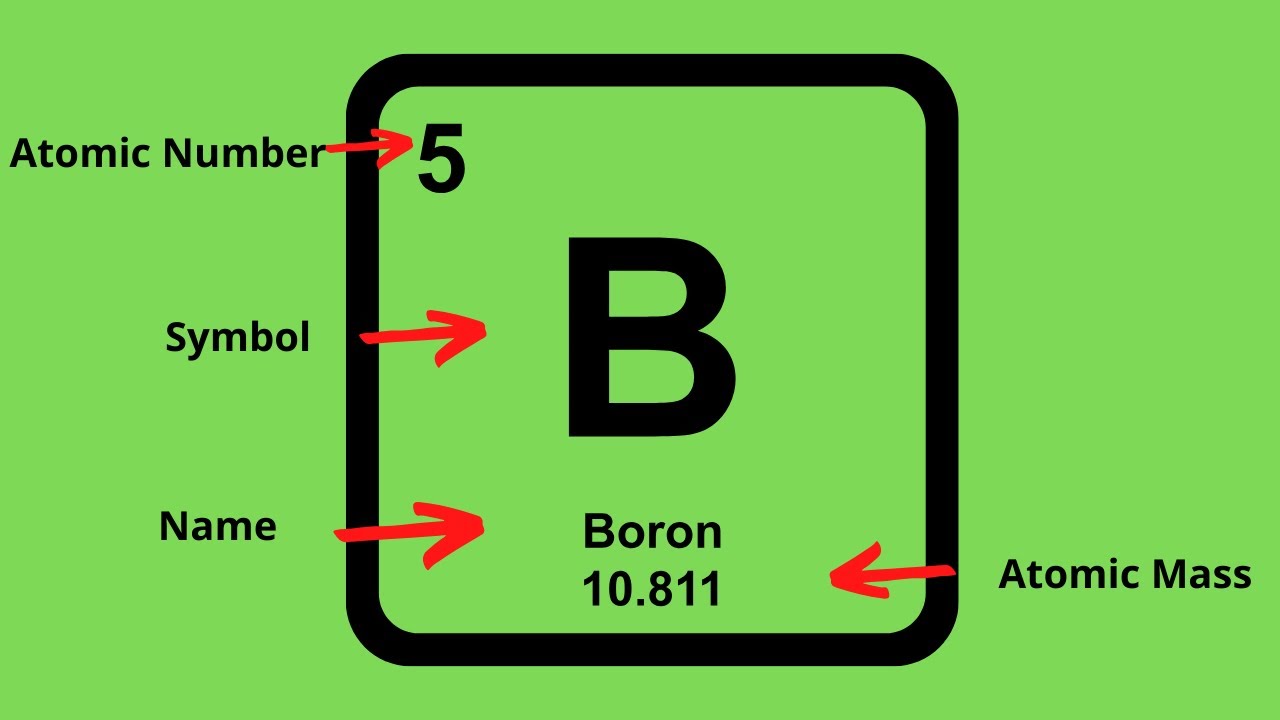
How To Find The Protons Neutrons And Electrons Of An Element On The Periodic Table Youtube
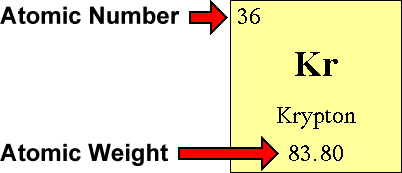
Questions And Answers How Do I Find The Number Of Protons Electrons And Neutrons That Are In An Atom Of An Element

How To Calculate The Number Of Protons Neutrons And Electrons Chemistry Youtube

How To Find The Number Of Protons Neutrons And Electrons
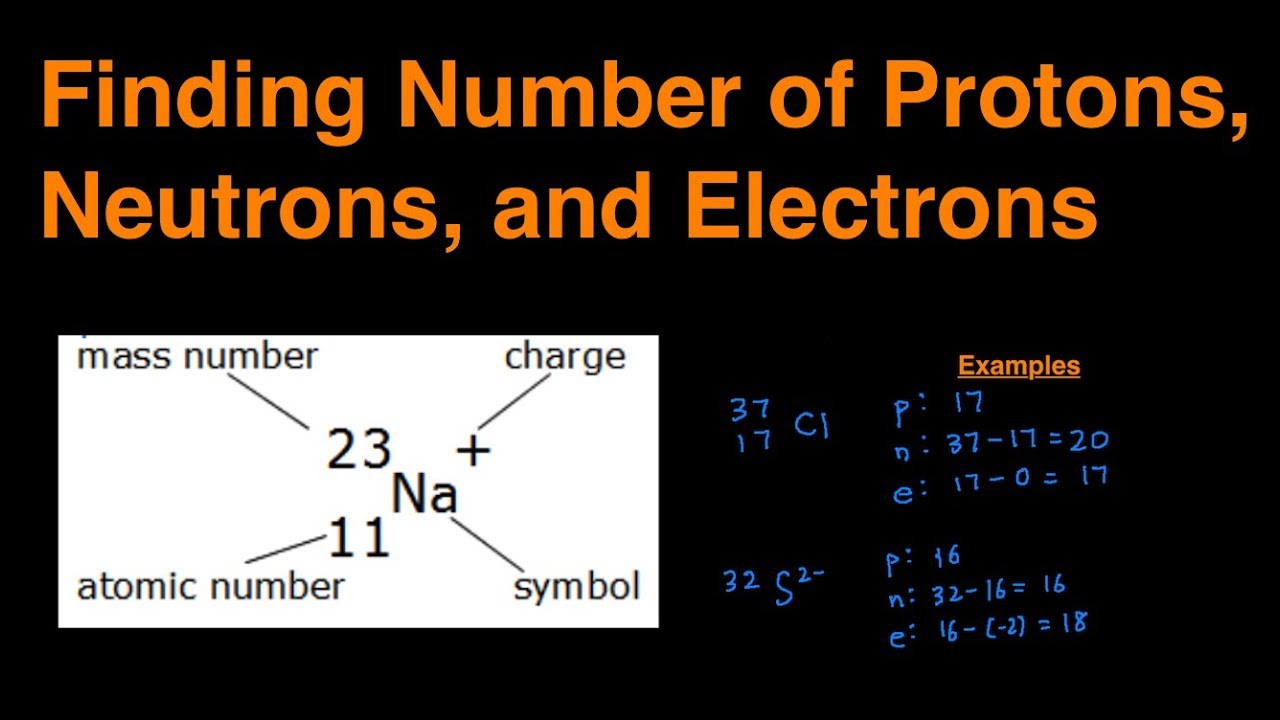
How To Determine Number Of Protons Neutrons And Electrons Step By Step With Examples Youtube

How To Find The Number Of Neutrons In An Atom 11 Steps
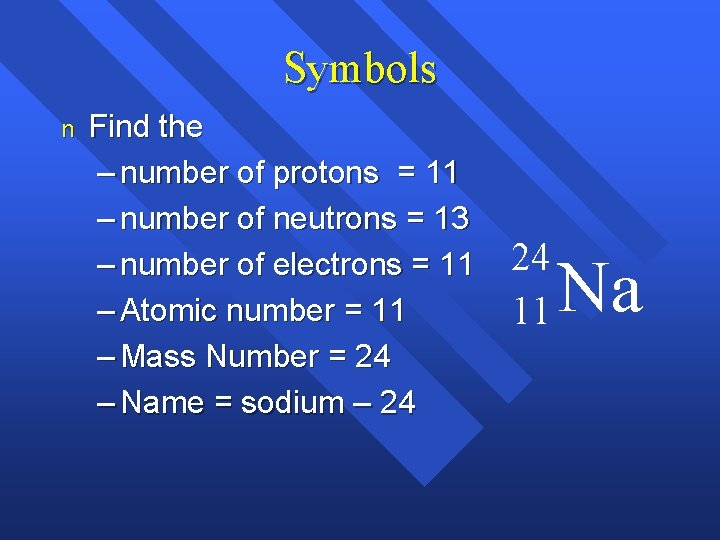
Determine The Number Of Protons Neutrons And Electrons
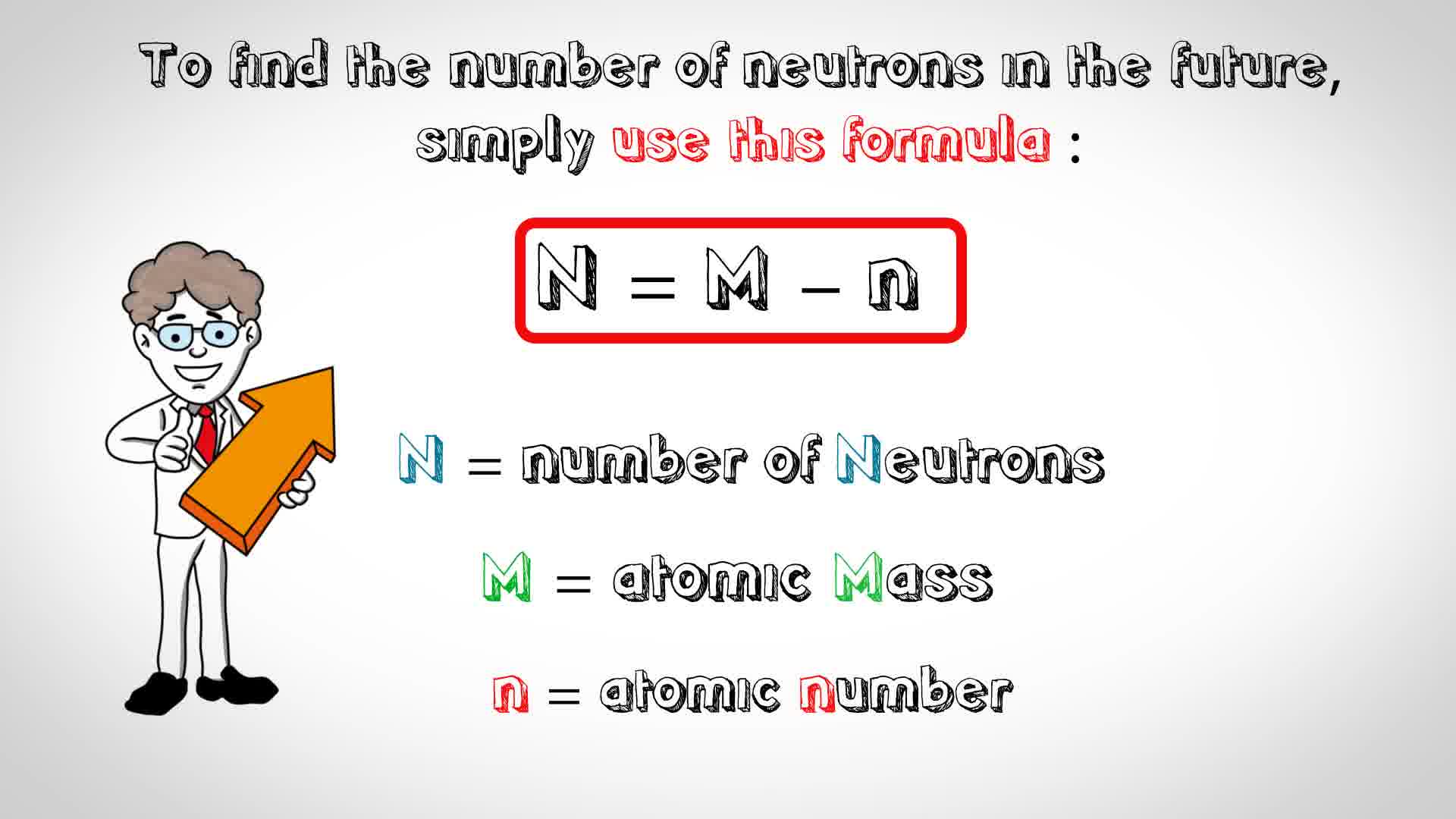
Zirconium Has An Atomic Number Of 40 How Many Neutrons Are There In An Isotope Of Zirconium 92 Socratic

Question Video Deducing The Number Of Neutrons From The Nuclide Notation And The Sorts Nagwa
Cell Phone Caller Id Private Number How To Find Number Of Neutrons Protons And Electrons In An Element
Post a Comment
Post a Comment